What Does a Coefficient Represent in a Chemical Formula
In other words it reflects how similar the measurements of two or more variables are across a. The coefficient tells us how many molecules of a given formula are present.
The purpose of coefficients in a chemical equation is that coefficients are used to show the ratio in which reactants combine and products for a chemical reaction.

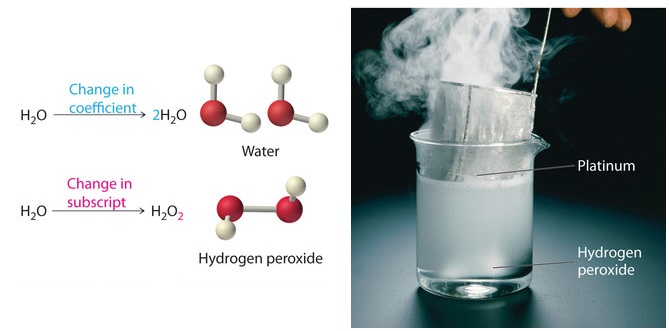
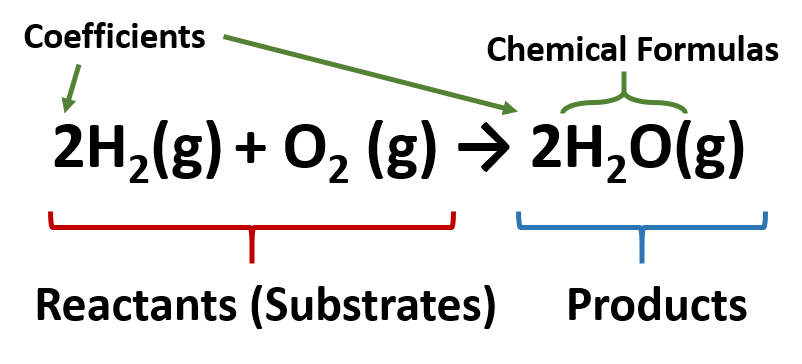
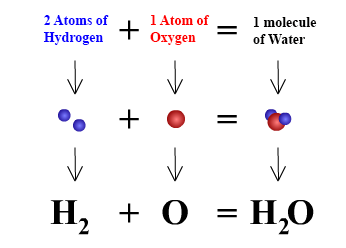
. A coefficient is a number placed in front of a chemical symbol or formula. For every two unit of hydrogen molecule you need one unit of oxygen molecule and the result is two unit of water molecule. Coefficients are used to balance chemical equations.
It can mean the number of atoms molecules formula units or moles. What does a coefficient represent in chemical formula. If a written chemical reaction were not balanced in this manner there would be no information available regarding the relationship.
If 2 A 3 B 4 C we know that the input of A and B is 23 in pieces A B and that we get a. The coefficients are a ratio. This tells us that two molecules of hydrogen H2 will react with one molecule of oxygen O2 to give two molecules of water H2O.
A correlation coefficient is a number between -1 and 1 that tells you the strength and direction of a relationship between variables. Coefficients are the numbers placed before the reactants in a chemical equation so that the number of atoms in the products on the right side of the equation are equal to the number of atoms in the reactants on the left side. When balancing a chemical equationthe law of conservation of matter must be upheld.
In Chemistry the coefficient is the number in front of the formula. A balanced chemical reaction means that the number of atoms of each element on both sides of the equation are the same. In Chemistry the coefficient is the number in front of the formula.
Write at least 20 characters to explain it well. Coefficients in a chemical equation represent the number of units of the formula immediately following the coefficient that are involved. The coefficients in chemical equations represent relative numbers.
There are 4 chromium atoms and 6 oxygen atoms on the right side of the equation. In chemistry a coefficient in front of a chemical formula tellsyou how many moles you have. It shows how many atoms or molecules of the substance are involved in the reaction.
To balance chemical equations coefficients are used. To do this you add coefficients as needed and these coefficients represent mole ratios of either reactants or products. The coefficient represents the number of atoms or molecules of the substance involved in the.
First they tell us what substances are reacting those being used up and what substances are products those being made. To do this youadd. Chemical equations give information in two major areas.
What does a coefficient represent in chemical formula. Correlation Coefficient Types Formulas Examples. The Meaning of a Chemical Equation.
It represents the amount of the substance. For example two molecules of. It shows how many atoms or molecules of the substance are involved in the reaction.
That unit is often the particle itself whether its an atom a metal ion a more substantial molecule or even a protein or enzyme. Coefficients are numbers placed in front of a compoundmolecule in a chemical reaction. The numerical coefficients in chemical equations show the ratio of the molecules of each substance involved in a chemical reaction both reactants and products.
Second the coefficients of a balanced equation tell us in what ratio the substances react or are produced. Published on August 2 2021 by Pritha BhandariRevised on December 2 2021.
7 4 How To Write Balanced Chemical Equations Chemistry Libretexts
Ch104 Chapter 5 Chemical Reactions Chemistry

Chemical Formula Definition And Examples
Nondestructive Evaluation Physics Atomic Elements
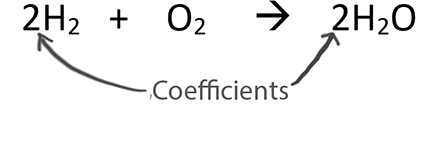
How To Balance Mg Oh 2 Hno3 Mg No3 2 H2o Breslyn Org

Reaction Stoichiometry Boundless Chemistry

Dublin Schools Lesson Mole Ratio And Coefficients

8 2 How Do We Represent Chemical Reactions Chemical Reactions Siyavula

What Is A Chemical Equation Definition Examples Video Lesson Transcript Study Com
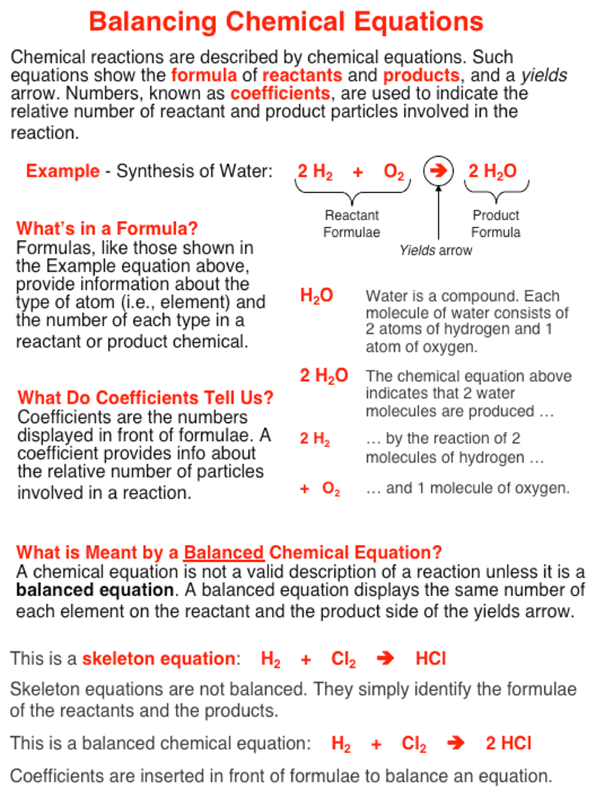
Balancing Chemical Equations Help

What Are Chemical Equations Detailed Explanation Examples

The Difference Between Coefficients And Subscripts In Chemical Equations Youtube
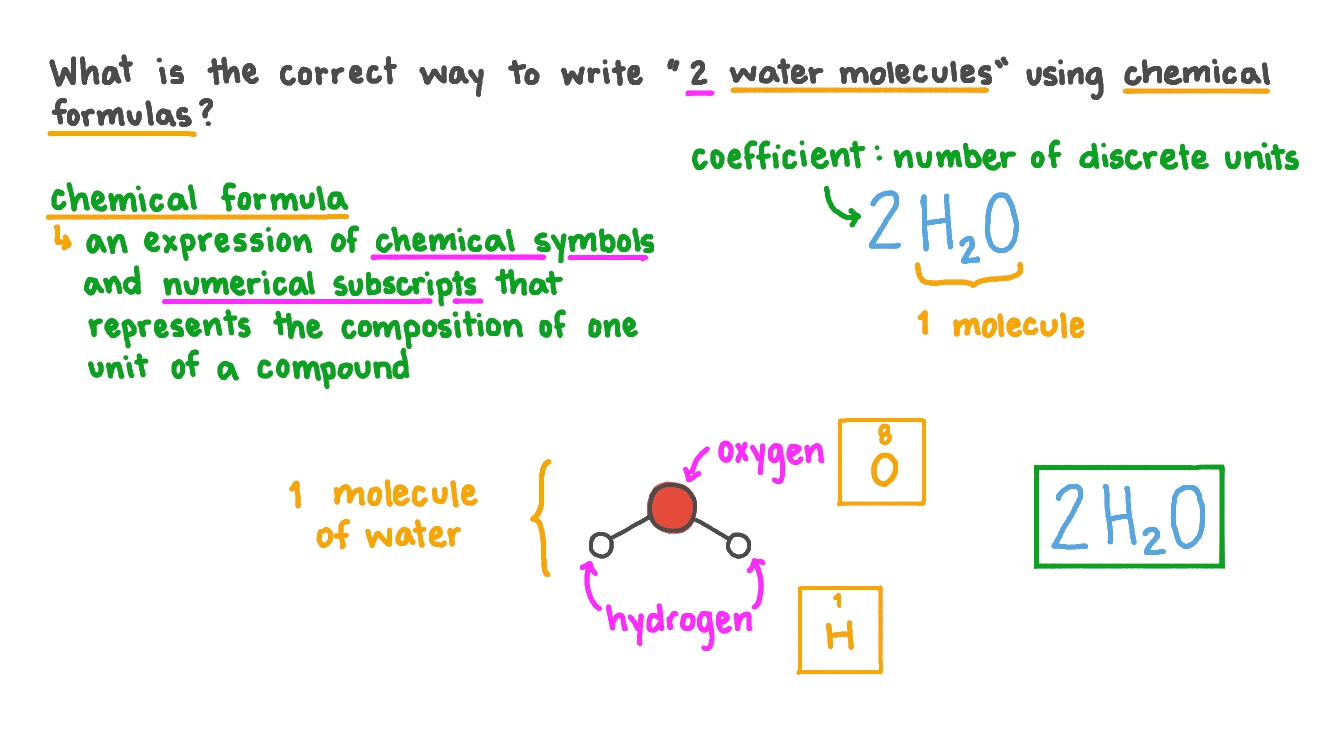
Question Video Expressing Numbers Of Molecules In Chemical Notation Nagwa

Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab





Comments
Post a Comment